Rarefied gases
Equation of state of rarefied reacting gases
Russian
Dense gases
Calculators of thermodynamic processes and simple gas dynamic flows
Thermodynamic parameters of individual components
Equilibrium chemical composition at a given temperature and density
Stationary shock waves (Hugoniot conditions)
Stationary detonation waves (Chapman-Jouguet parameters), mixture analysis
Combustion (v=const)
Combustion (p=const)
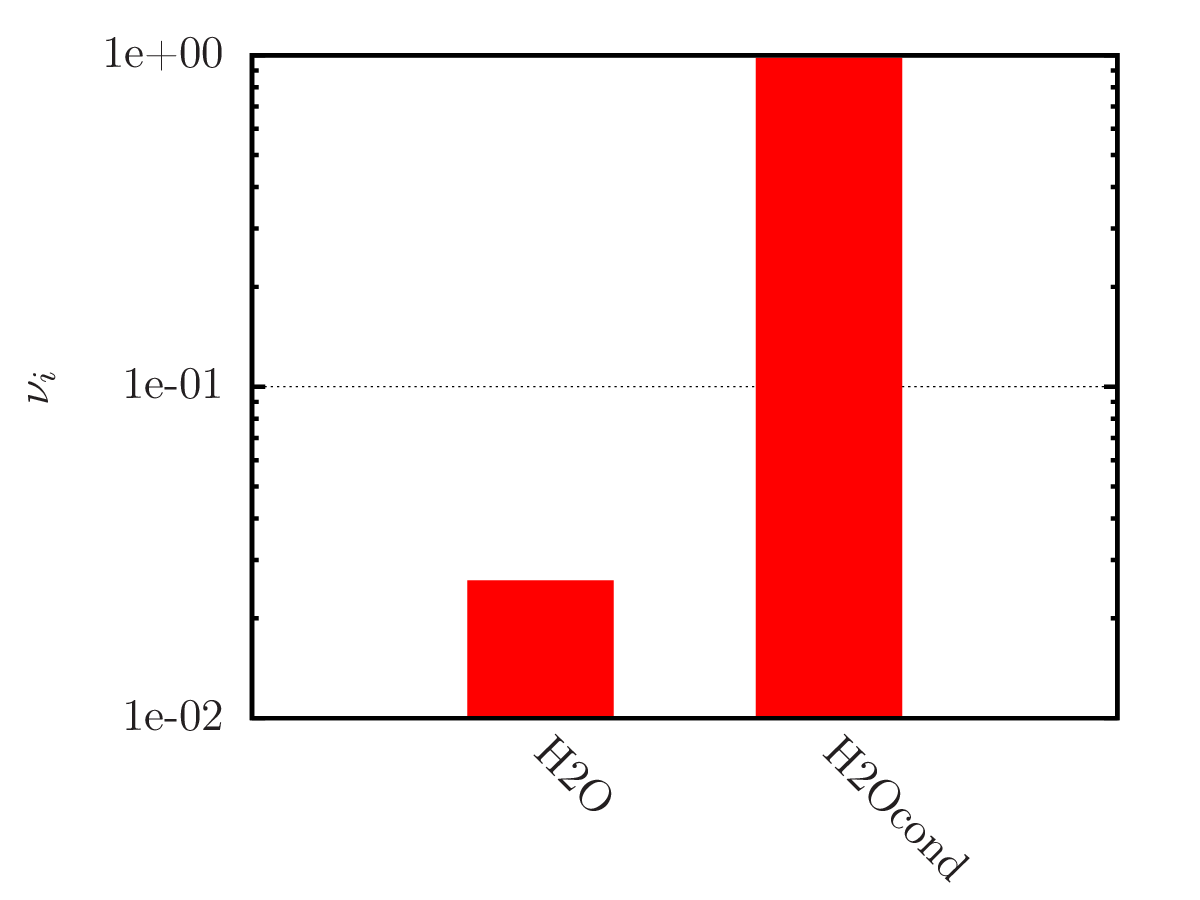
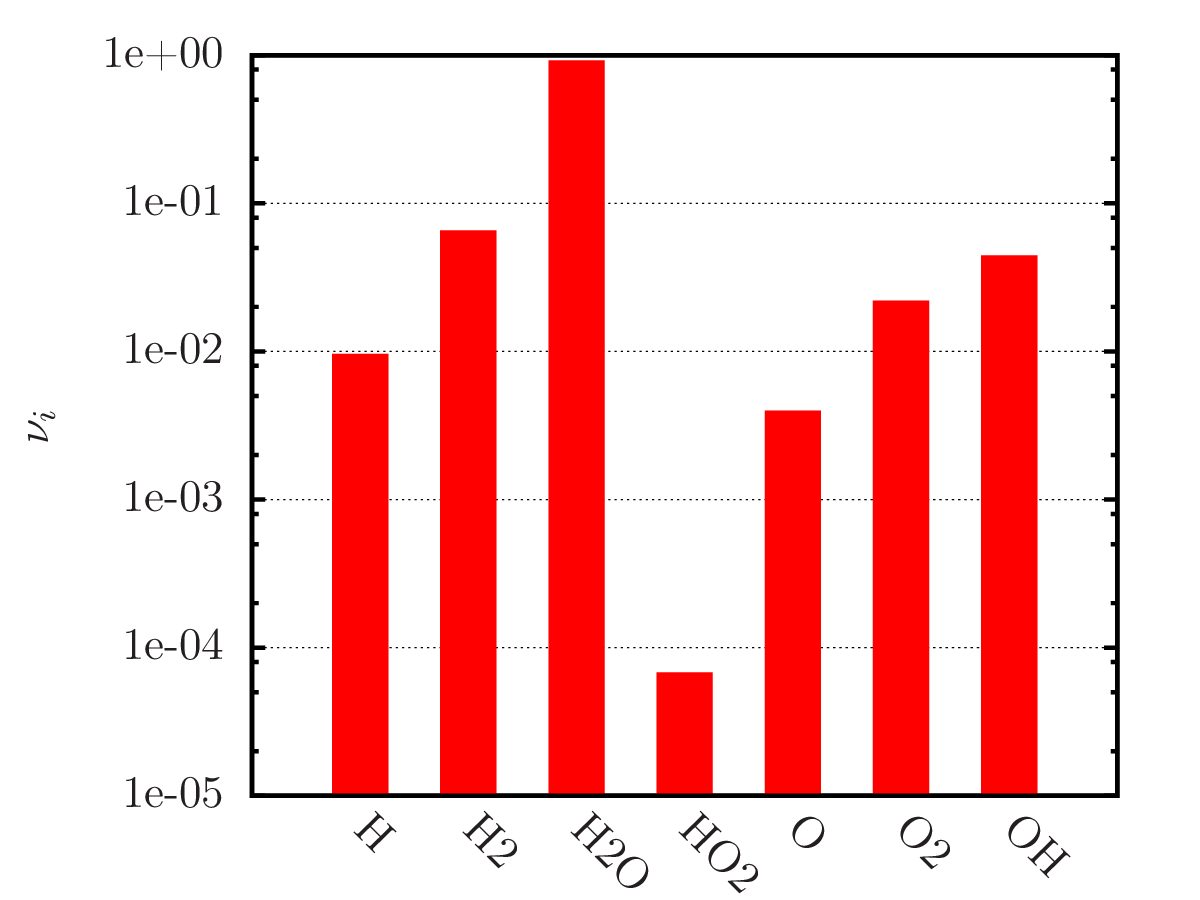
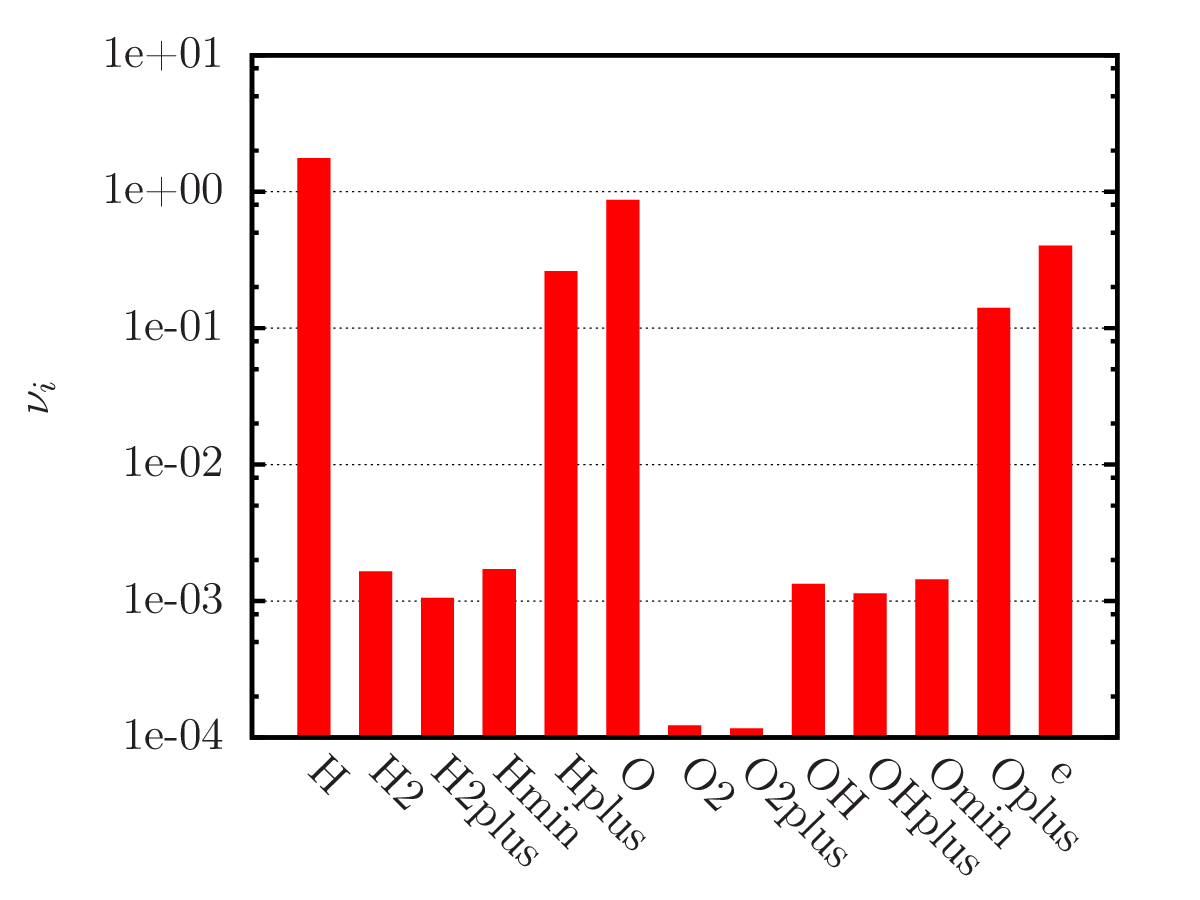
An example of calculating the equilibrium chemical composition of a mixture 2H + O (water) at temperatures of 300, 3 000 and 20 000 K. Density 1.3 kg/m3.
Brief description
Using the methods of statistical physics, a model of thermodynamics of a reacting mixture of rarefied gases and condensed components is realized. To determine the detailed equilibrium chemical composition, the NVT ensemble is considered and the minimum free energy of the mixture of all possible components is numerically found. To determine the enthalpies and free energies of chemical compounds, tabular data are used (V.P. Glushko, Stall D.).
The software package has several levels of use: a web interface (http://ancient.hydro.nsc.ru/chem), which allows for easy online calculations of the thermodynamic parameters of reacting gases and a number of simple gas-dynamic flows; a library in the c ++ programming language for embedding into other applications and a database of thermodynamic characteristics of chemical components.
The resource was developed with the support of the Russian Foundation for Basic Research (RFBR 12-01-00177-a).
Features
- Calculation of the equilibrium chemical composition of a gas mixture based on the elements He, Ne, Ar, Kr, Xe, H, C, N, O, Na, Mg, Al, Fe, Si, S in the temperature range 200 - 20,000 K.
- Calculation of a number of thermodynamic parameters: pressure, enthalpy, internal energy, heat capacity, equilibrium and frozen adiabatic exponent.
- Plotting equilibrium and frozen shock adiabats.
- Determination of thermodynamic parameters of combustion at v = const and p = const.
- Determination of parameters of stationary detonation waves.
- The possibility of the formation of condensed phases C, H2O, Na, Na2O, Mg, MgO, Al, Al2O3, S, SiO2, Si, Fe, FeO, Fe2O3, Fe3O4, FeS, FeS2 is taken into account.
The implemented approach is successfully applied to quantitatively predict the energy and mechanical characteristics of intense flows. To determine the fire and explosion hazard of mixtures of combustible gases and gas suspensions. For numerical calculations of gas-dynamic flows with a real equation of state.
Documentation
- Rare gases pdf.
- Dense gases (ru, en).
- Certificates of registration of computer programs:
- Pruuel E.R., Vasiliev A.A., Kashkarov A.O. Method for calculating equilibrium shock and detonation waves in reacting gases. Certificate of state registration of a computer program No. 2018660109, 2018 pdf .
- Pruuel E.R. Method for calculating the equilibrium chemical composition and thermodynamic parameters of dense gases. Certificate of state registration of a computer program No. 2019666436, 2019. pdf .
- Pruuel E.R., Anisichkin V.F. Method for calculating equilibrium shock adiabats in dense gases. Certificate of state registration of a computer program No. 2019666437, 2019. pdf .
- Album of detonation adiabats pdf .
How to link
- Pruuel E.P., Vasilev A.A. Equation of State of Gas Detonation Products. Allowance for the Formation of the Condensed Phase of Carbon // Combustion, Explosion, and Shock Waves. — 2021. — Т. 57, No 5. — С. 1—11. — DOI: 10.1134/S0010508221050075, pdf.
- Прууэл Э. Р., Васильев А.А. Уравнение состояния продуктов газовой детонации. Учет формирования конденсированной фазы углерода // Физика горения и взрыва. - 2021. -т. 57, N 5. С 74-85. — DOI: 10.15372/FGV20210507, pdf.
Useful links
- Ivantermo database http://www.chem.msu.su/rus/handbook/ivtan/welcome.html.
- Thermodynamic properties of individual substances. Edited by V.P. Glushko 3rd edition:
- Volume 1 (B1, B2) 1978 year. (O, H(D, T), F, Cl, Br, I, He, Ne, Ar, Kr, Xe, Rn, S, N, P);
- Volume 2 (B1, B2) 1979 year. (C, Si, Ge, Sn, Pb);
- Volume 3 (B1, B2) 1981 year. (B, Al, Ga, In, Tl, Be, Mg, Ca, Sr, Ba);
- Volume 4 (B1, B2) 1982 year. (Cr, Mo, W, V, Nb, Ta, Ti, Zr, Hf, Sc, Y, La, Th, U, Pu, Li, Na, K, Rb, Cs);
- Volumes 5, 6 http://www.chem.msu.su/Zn (Zn, Cu, Fe, Co, Ni, Mn, Cr, V, Ti, Sc).
- National Institute of Standards and Technology (USA) https://webbook.nist.gov/chemistry.
- The chemical thermodynamics of organics compounds. Daniel R. Stull. 1969 year. 807 pages. pdf.
- NASA online CEA. https://cearun.grc.nasa.gov.
- FactSage web. https://www.crct.polymtl.ca/factweb.php.
- Rusbank. Shock wave database. href="http://www.ihed.ras.ru/rusbank.
- Sources of information on the thermodynamic properties of substances on the Internet. G.V. Belov. 2013 г. pdf.
- Thermodynamic databases and software systems for thermodynamic modeling pdf.
- LASL SHOCK HUGONIOT DATA. Stanley P. Marsh. pdf.
- LASL EXPLOSIVE PROPERTY DATA. pdf.
- Pyro help. pdf.
- Detonation properties of condensed explosives computed using the Kihara-Hikitata-Tanaka equation of state. Tanaka. pdf.
- Замалин В. М., Норман Г. Э., Филинов В. С. Метод Монте-Карло в статистической термодинамике — М. : Наука, 1977. pdf.
Collaboration
Interested in cooperation:
- with specialists in the field of chemistry to test and check the operation of the complex;
- with specialists in the field of quantum calculations of the energy characteristics of chemical compounds to expand the database;
- with specialists in numerical methods for conducting gas-dynamic calculations of intense flows;
- with IT specialists to develop a python interface and extend functionality.
Authors
Pruuel Eduard Reinovich ([email protected]), Kashkarov Aleksey Olegovich, Bondarenko Tatiana Andreevna.
M.A. Lavrent'ev SB RAS


